CONTER CURRENT EXTRACTION
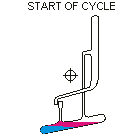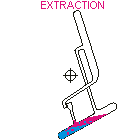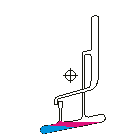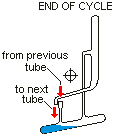
Countercurrent Extraction - Craig Apparatus. Theory A method of multiple liquid-liquid extractions is countercurrent extraction, which permits the separation of substances with different distribution coefficients (ratios). A clever design known as Craig apparatus is used for this purpose (Lyman C. Craig, 1943). Craig apparatus consists of a series of glass tubes (r: 0, 1, 2..) that are designed and arranged such that the lighter liquid phase is transferred from one tube to the next. The liquid-liquid extractions are taking place simultaneously in all tubes of the apparatus which is usually driven electromechanically. In the following animated picture of a single glass tube the typical "extraction/transfer" cycle is shown. The lower (heavier) phase of the two-phase solvent system (e.g. water, blue layer in the picture) is the "stationary phase", whereas the upper (lighter) phase (e.g. hexane, red layer in the picture) is the "mobile phase". In the begin...